INFORM SMA
Current projects within the INFORM SMA research theme are described below.
Leadership: Dr. Maryam Oskoui
National clinical trial currently underway
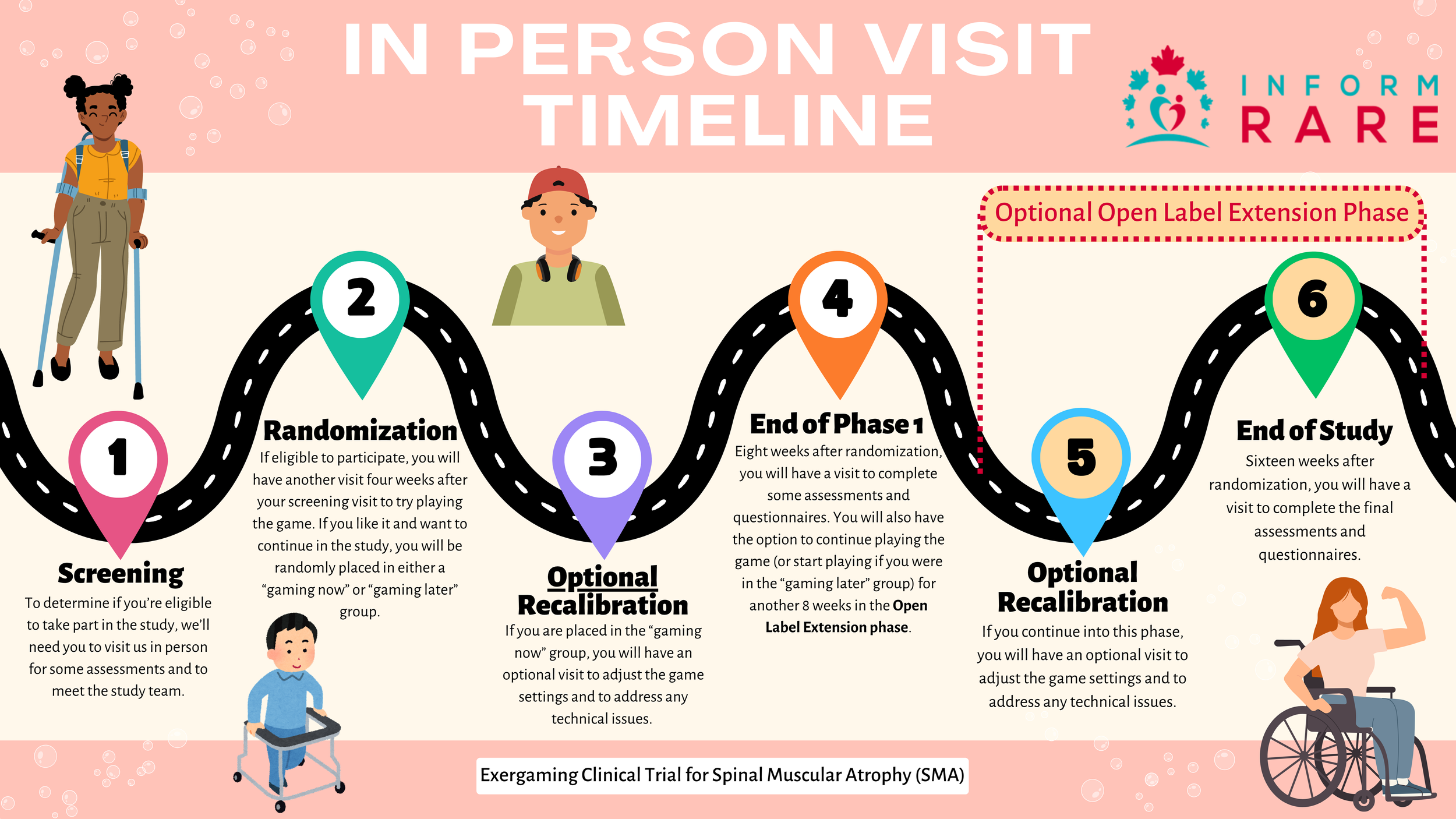
We are currently recruiting for a randomized clinical trial testing the effectiveness of a custom-developed exergame to promote physical activity in children and youth with SMA.
Interactive computer-based platforms such as exergames offer exciting possibilities for home-based rehabilitation. In collaboration with youth with SMA, parents/caregivers, physiotherapists, physicians, and data scientists from F. Hoffmann-La Roche Ltd, we have co-developed an exergame focusing on upper limb and trunk movements in youth with neuromuscular disorders. The exergame uses the Microsoft Azure Kinect to capture body movements and measure progress.
We are now undertaking a study to evaluate the impact of the 8-week home-based exergaming intervention, as compared to usual care, on performance of everyday activities in children and youth with SMA.
Where is this happening?
The trial is taking place in 5 centres across Canada. IWK Health Services in Halifax, Alberta Children’s Hospital in Calgary, Centre de readaptation Marie-Enfant in Montréal, Hospital for SickKids in Toronto and BC Children’s Hospital in Vancouver.
This video provides more information about the research study:
What does the study involve?
During this trial you will also have to wear the Syde device, which is a wearable activity monitor. This video provide more information about the Syde:
This trial is supported by a Clinical Trials operating grant from the Canadian Institutes of Health Research (CIHR). The trial details (NCT06396325) are available here: https://clinicaltrials.gov/study/NCT06396325
Interested in joining? Please contact Ariane Belzile, our project manager at ariane.belzile@muhc.mcgill.ca
Optimizing the Canadian SMA Registry for This Trial
To optimize the Canadian SMA Registry for trial readiness, we have strengthened our data management and governance structure by leveraging the LASSO platform with experienced developers and data managers. LASSO is a web-based research data management platform designed to store, cross-link, curate, visualize and share many types of research data, ideally suited to manage large multi-modal multi-site datasets acquired over time in a longitudinal study.
We are presently working to streamline the data collected within the Canadian SMA Registry to ensure alignment with other international SMA registries.
Development of a Core Outcome Set
The INFORM SMA research team, together with national experts (including patients, caregivers, patient organizations, healthcare providers, policy advisors, payers, and industry representatives) has build on the TREAT-NMD SMA Core Dataset to determine how best to measure the outcomes in the core outcome set. The results of this process will finalize the recommended core outcome measurements and definitions for children and adolescents with SMA across different ages and functional abilities. This will be integrated into the Canadian SMA Registry. Two rounds of a survey using the Delphi consensus methodology and a virtual consensus workshop were conducted. The results will soon be published.
Development of the Exergame
To inform the design of the exergame, we conducted online surveys and semi-structured interviews with patient groups and physiotherapists to identify needs, preferences, and barriers to implementation. The development phase consisted of an iterative process in which physiotherapists provided feedback to ensure that exercises were transformed into safe and intuitive movements to be performed within the game. Youth advisors with rare disease lived experience were also consulted to ensure that, in addition to exercise and customization requirements, game elements were focused on creating an enjoyable and engaging game.
Development of the Clinical Trial
We have completed a feasibility study for this exergame across two Canadian sites (Montreal & Vancouver). The primary goal was to assess the usability and acceptability of the exergame in youth with SMA and neurotypical peers. In 2025, the results were published in Muscle and Nerve.
The trial uses the optimized Canadian SMA Registry to support enrollment and data collection. We hope that this registry-based design can overcome the common constraints of randomized trials in small patient populations that have led to a comparative paucity of randomized trials in pediatric rare disease populations, including SMA.